Some flowers in their individual development process must require a low temperature cycle to promote flower bud formation and floral development. This process is called the vernalization stage. The cold stimulation and treatment process that causes flowers to pass through the vernalization stage is called spring. Effect. Vernalization is a must-have program for some flowers to flower again, and is very important in flower production. Different flowers and different types of the same flower have different requirements for low-temperature vernalization. For example, the autumn sowing flowers planted in the open field are subjected to vernalization during the overwintering period. However, many flowers on the market today promote flower bud differentiation by artificially lowering the temperature. Some flowers must be treated at low temperatures for a period of time when the seeds are germinating, while others are treated at low temperatures when the seedlings are spread out. The treatment temperature and processing time for vernalization vary with the type of flower. In general, the spring flower requires 5 to 15 days at a temperature of 5°C~12°C, such as annual flowers and autumn flowering perennial grass flowers; Winter flower requires 30~70 at a temperature of 0°C~10°C. Days, such as biennial flowers, perennial flowers that bloom in early spring; semi-winter flowers are required to be treated at a temperature of 3°C to 15°C for 15 to 20 days. The flowers that have not yet completed vernalization are returned to normal temperature, and the vernalization effect disappears.
1.Good safety of gelatin-free.The first lyophilized Varicella Vaccine, containing no gelatin from animals, invented and produced in China. Getting rid of gelatin from varicella vaccine can significantly decrease the ratio of anaphylaxis incidence .
2.Long validity period by good stability. The first approved varicella vaccine with 36 months of validity period in the world. Adopting BH-2 stabilizer with own IP rights (Chinese patent granted No.: ZL200910138411.6, International patent application No.: PCT/CN2009/001405) greatly enhances the stability of the product and ensures the validity period for 36 months under the storage condition.
3.Better protection with high titer and immune eff Varicella Vaccine(Live) Live Bioproducts Pharmaceutical,Live High Potency Medicine,Live Preventive Pharmaceutical,Varicella Biopharmaceutical Live Changchun BCHT Biotechnology Co.,Ltd , https://www.ccbcht.net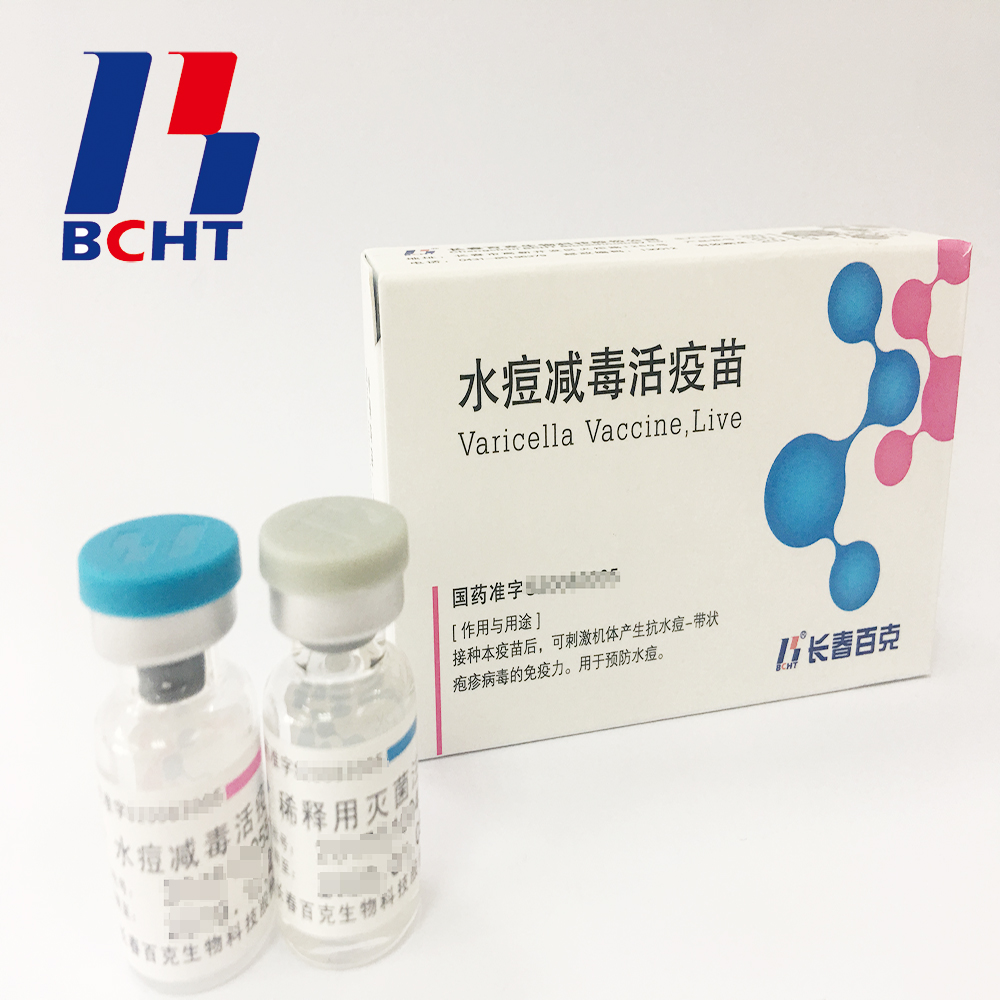 icacy. It`s documented that compared with the varicella vaccine of low titer, high titer vaccine can reduce breakthrough cases by nearly 75% in vaccination. Of all released batches of Varicella Vaccine from BCHT, the titers are not less than 10000 PFU/dose tested by National Institutes for Food and Drug Control.
icacy. It`s documented that compared with the varicella vaccine of low titer, high titer vaccine can reduce breakthrough cases by nearly 75% in vaccination. Of all released batches of Varicella Vaccine from BCHT, the titers are not less than 10000 PFU/dose tested by National Institutes for Food and Drug Control.
How do flowers perform vernalization
Next Article
Pea brown spot
Prev Article
Diagnosis and Treatment of Cattle Crows