USE: Treatment of infections of lower respiratory tract including pneumonia, urinary tract, skin and skin structures, bone and joints; treatment of bacteremia/septicemia, CNS infections, intra-abdominal infections including peritonitis, gynecological infections including pelvic inflammatory disease USE: Treatment of infections of lower respiratory tract including pneumonia, urinary tract, skin and skin structures, bone and joints; treatment of bacteremia/septicemia, CNS infections, intra-abdominal infections including peritonitis, gynecological infections including pelvic inflammatory disease
CAS NO
64485-93-4
MF
C16H16N5NaO7S2
EINECS No
264-915-9
Place of Origin
China (Mainland)
Assay
no less than 99%
Packaging Details
25kg drum
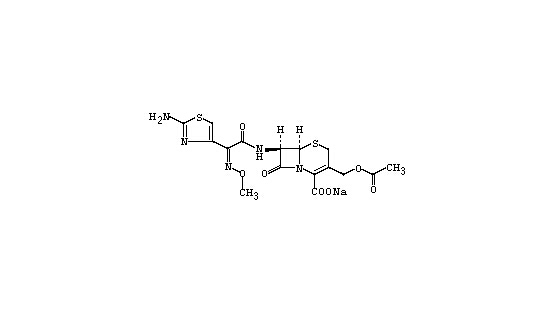

Appearance: This product is white, almost white or yellowish white crystalline powder; odorless or slight special smell. It is free soluble in water, slightly soluble in ethanol, insoluble in chloroform.
Â
SPECIFICATION:
Product Name
Cefotaxime Sodium Sterile
Package
6kg/tin, 2tins/carton
Appearance
White to slightly yellow crystal or powder
Solubility
Freely soluble in water; sparingly soluble in ethanol
Identification
Infrared-spectrometry:both spectra exhibit similar intensities of absorption at the same wave numbers
It responds to the qualitative test of sodium salt
The chromatogram of the Assay obtained as directed in the Assay exhibits a major peak for cefotaxime, the retention time of which corresponds to that exhibited in the chromatogram of the Standard preparation
obtained as directed in the Assay
Specific rotation
+58°~ +64°
pH
4.5~6.5
Clarity of solution
Clear
Clarity of solution
Absorbance : ≤0.20
Loss on drying
≤3.0%
Particulate matter
≥10μm, ≤ 3000
≥25μm, ≤ 300
Chromatographic purity
Any individual impurity: ≤1.0%
Total impurities:≤3.0%
Bacterial endotoxins
Not more than 0.025 USP Endotoxin Unit per mg
Sterility
Should be sterile
Assay
916ug/mg~964ug/mg
Visible foreign matter
Essentially free from
Labeling
According to normative documentation
CAS NO
64485-93-4
MF
C16H16N5NaO7S2
EINECS No
264-915-9
Place of Origin
China (Mainland)
Assay
no less than 99%
Packaging Details
25kg drum


Appearance: This product is white, almost white or yellowish white crystalline powder; odorless or slight special smell. It is free soluble in water, slightly soluble in ethanol, insoluble in chloroform.
Â
SPECIFICATION:
Product Name
Cefotaxime Sodium Sterile
Package
6kg/tin, 2tins/carton
Appearance
White to slightly yellow crystal or powder
Solubility
Freely soluble in water; sparingly soluble in ethanol
Identification
Infrared-spectrometry:both spectra exhibit similar intensities of absorption at the same wave numbers
It responds to the qualitative test of sodium salt
The chromatogram of the Assay obtained as directed in the Assay exhibits a major peak for cefotaxime, the retention time of which corresponds to that exhibited in the chromatogram of the Standard preparation
obtained as directed in the Assay
Specific rotation
+58°~ +64°
pH
4.5~6.5
Clarity of solution
Clear
Clarity of solution
Absorbance : ≤0.20
Loss on drying
≤3.0%
Particulate matter
≥10μm, ≤ 3000
≥25μm, ≤ 300
Chromatographic purity
Any individual impurity: ≤1.0%
Total impurities:≤3.0%
Bacterial endotoxins
Not more than 0.025 USP Endotoxin Unit per mg
Sterility
Should be sterile
Assay
916ug/mg~964ug/mg
Visible foreign matter
Essentially free from
Labeling
According to normative documentation
USP Cefotaxime Sodium for Anti-Inflammatory Use
Model NO.: USP
Assay: 940ug/Mg
pH: 4.5-6.5
Loss on Drying: 3.0% Max
Trademark: REACHEVER
Transport Package: 25kg/Drum
Specification: GMP
Origin: China(Mainland)
Model NO.: USP
Assay: 940ug/Mg
pH: 4.5-6.5
Loss on Drying: 3.0% Max
Trademark: REACHEVER
Transport Package: 25kg/Drum
Specification: GMP
Origin: China(Mainland)
ANTIBACTERIAL ANTI-INFLAMMATORY USE -CEFOTAXIME SODIUMNext Article
Raw Material Ivermectin for Veterinary Drugs