Omeprazole, is a medication used in the treatment of gastroesophageal reflux disease, peptic ulcer disease, and Zollinger-Ellison syndrome.It is also used to prevent upper gastrointestinal bleeding in people who are at high risk. It can be taken by mouth or injected into a vein. Common side effects include nausea, vomiting, headaches, and increased intestinal gas. Serious side effects may include Clostridium difficile colitis, an increased risk of pneumonia, an increased risk of bone fractures, and the potential of masking stomach cancer. It is unclear if it is safe for use in pregnancy. Omeprazole is a proton pump inhibitor and as such blocks the release of stomach acid.



Â
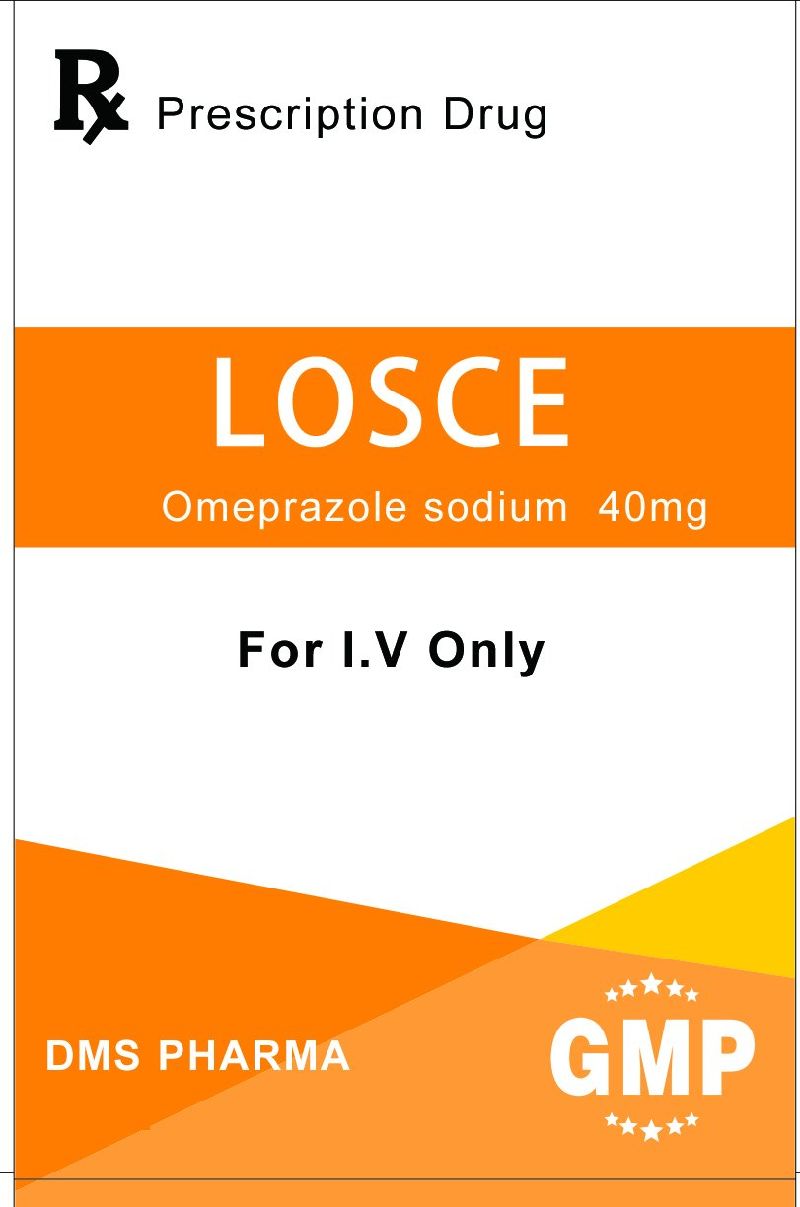
Product name
LOSCE
Contents
Omeprazole sodium 40mg
Formeulation
Powder For Injection
Package
1 vial and 1Â AMP(SOLVENT)
Application
Anti-Uler
WE CAN PROVIDE MAKE FORMEULATION (OEM)
Service we can provide:
1. Mixed container, we can mix different items in one container.
2. Quality control, before shipment, free sample for test. After shipment, keep sample for 3Â years
3. Prompt shipment with professional documents
4. Packing as your request, with photo before shipment.
Â
Guyenne Delayed Release, Acid Reducer, Omeprazole Injection 40 Mg GMP Factory